Wegovy: Cost, Efficacy, Side effects of The New Semaglutide!


Table of Contents
- Everything You Need to Know About Wegovy
- What Is Wegovy?
- What Is The Active Ingredient?
- How Does Wegovy Work?
- Wegovy Benefits
- What Can Wegovy Do in Chronic Weight Management?
- Who Can Take Wegovy?
- Multiple Endocrine Neoplasia Type 2
- How Much Does Wegovy Cost?
- How Much Weight Loss Will I Get When I Use Wegovy?
- Does Insurance Cover Wegovy?
- Cost of Wegovy with Insurance
- Is Wegovy FDA-Approved?
- What is Included in The FDA Label of Wegovy?
- Indications and Usage
- Limitations of Use
- Important Administration Instructions
- Contraindications
- Warnings and Precautions
- Clinical Trials Experience
- Drug Interactions
- Use in Specific Groups
- Overdose
- Clinical Pharmacology
- Others
- How Do I Take My Wegovy?
- Wegovy Side Effects
- Common Side Effects
- Mild Side Effects
- Contraindications of Wegovy
- Some Health Problems Caused by Wegovy
- Thyroid C-cell Tumors
- Hypoglycemia
- Pancreatitis
- Diseases of the Gallbladder or Gallstones
- Diabetic Retinopathy in Type 2 Diabetes
- Acute Kidney Injury
- Wegovy vs. Other Weight Loss Drugs and Therapies
- Should I tell my Doctor Before Using Wegovy?
- Conclusion
- About The Author
- Who is Dr. Ergin?
- REFERENCES:
Everything You Need to Know About Wegovy
In our world today, obesity is perhaps one of the most notable diseases that require extra care and long-term management. This chronic disease can be linked to several health complications that lessen life expectancy. Some complications related to obesity include cardiovascular diseases, diabetes, obstructive sleep apnea, and certain malignancies.
Even the current pandemic revealed that obesity is another factor in determining the severity of COVID-19. Can Wegovy be a new hope? Is Wegovy expensive? What are the side effects of Wegovy? We will touch on all these in this blog post. To combat the problem, physicians and researchers came up with several ways.
Different weight loss programs and various diets and exercise regimens were introduced. However, even with the success of these techniques, medical interventions are still necessary to strengthen the effect on obese individuals.
Medications with the potential to help treat obesity are beneficial, especially today, where obesity is rampant and is even considered a significant public health issue. Thankfully, a new weight loss medicine, Wegovy, by the pharmaceutical company Novo Nordisk has been recently approved by the Food and Drug Administration (FDA).
What Is Wegovy?
Wegovy
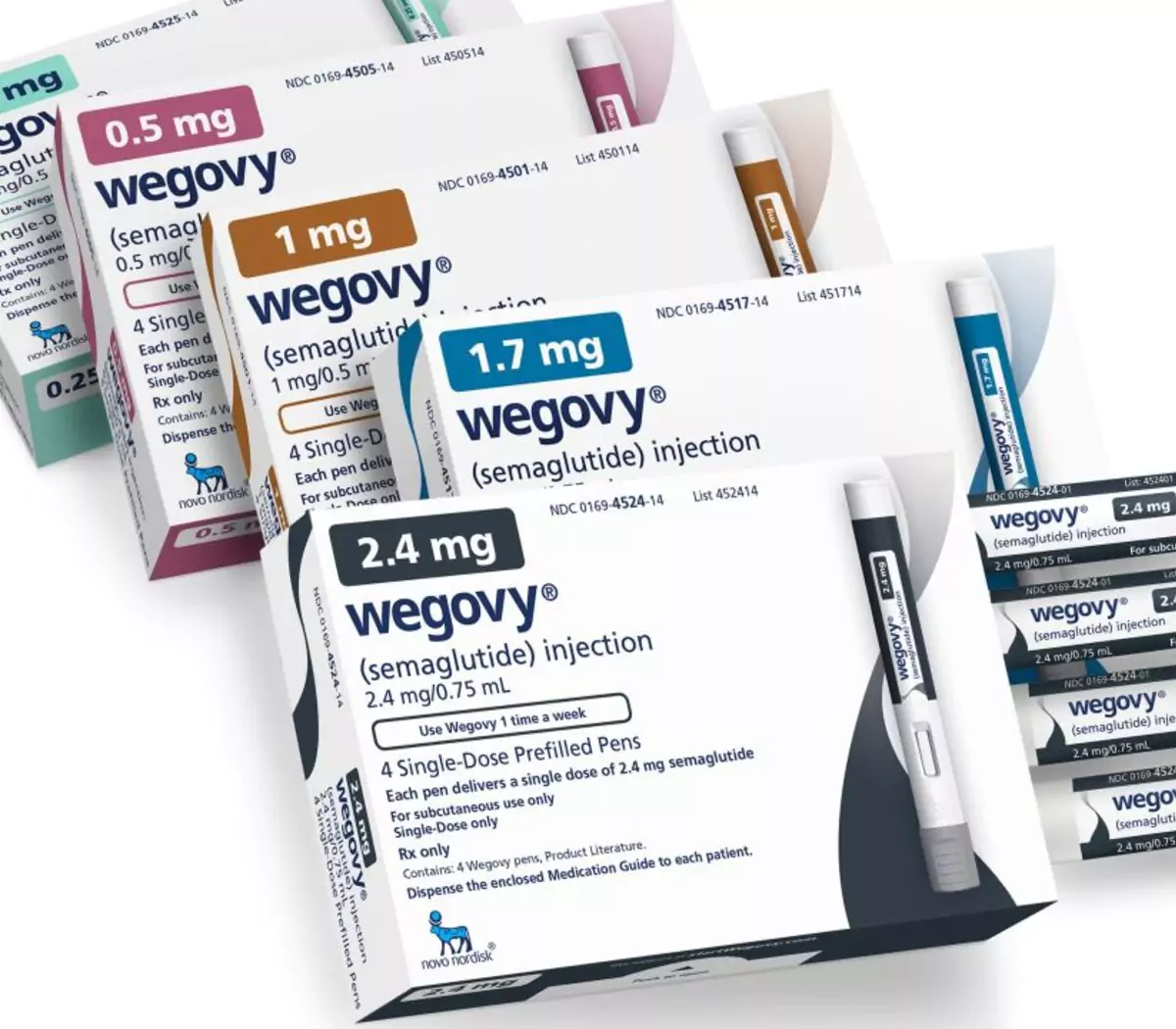
Wegovy is the newest weight loss drug to help individuals with obesity. It is manufactured by Novo Nordisk, one of the most notable Danish pharmaceutical companies. This weight loss drug specifically aims to assist in the chronic weight management of adults with obesity or overweight individuals with weight-related comorbidities like hypertension, hyperlipidemia, and diabetes. This medication helps hasten weight loss accompanied by the proper diet and physical activity.
What Is The Active Ingredient?
The active ingredient in Wegovy is semaglutide. This weight-loss medicine is a known GLP-1 agonist, and its mechanism of the action centers on mimicking GLP-1, a hormone produced in the body. Approximately, Wegovy has a 94% similarity with GLP-1.
semaglutide
Wegovy acts on several sites in the body, and one of the main targets is the pancreas. It induces the pancreas to release increased insulin levels to help improve the glucose uptake in the body. Wegovy also acts on the receptors in the brain, specifically the hypothalamus and brainstem.
This action leads to suppression of appetite that will cause you to eat a lesser amount of food than usual. This hormone also acts on the gastrointestinal tract by decreasing acid secretion and slowing food movement during digestion.
The weight loss resulting from the intake of Wegovy is by the mechanism of reducing the hunger felt by the user and increasing the sensation of fullness. Therefore, users tend to eat fewer foods and reduce their calorie intake. Of course, for this weight loss drug to be effective, the user should follow proper physical activity and diet.
How Does Wegovy Work?
Wegovy or its active substance belongs to the class of drugs known as Incretin mimetics. They work like or mimic the Incretin hormone, Glucagon-Like Peptide 1 (GLP-1). The Incretin hormones are secreted cells in our guts, including the stomach and intestines.
The hormone stimulates insulin production and blood sugar reduction by stimulating the pancreas. The Incretin hormone or the GLP-1 can also act on the brain to regulate your appetite and control hunger, which can help with long-term weight loss.
Wegovy and other medications that act like GLP-1 work by binding to the stomach cell receptors. This action helps slow the stomach-emptying process and makes you stay fuller for longer. The decreasing rate of hunger and staying fuller for longer make Wegovy great for weight management.
Wegovy is best used with foods or diets with low-calorie content and lots of physical activities. The combined effects of this lead to long-term weight loss.
- Wegovy is best for an adult who has a Body Mass Index (BMI) of:
- 30 kg/m2 or higher, which is the BMI for obesity.
- 27 kg/m2 or higher (the BMI for overweight individuals) with at least one weight-related medical
- condition, like abnormal blood lipids, high blood pressure, and type 2 diabetes.
Wegovy Benefits
Wegovy is very useful because it has lots of benefits. However, among this plenty of benefits is that Wegovy is very effective for managing weight. According to the United States Food and Drug Administration, it is reported in the Draft Guidance that medications used for weight management are required to show a significant amount of weight loss that is higher than that of a placebo.
The standard is set at 5% or greater than that, but in the case of Wegovy, it was more than 10%. In some clinical trials of Wegovy, it was reported that most participants or treated patients experience an average weight loss greater than 10% and almost 16%.
Another benefit is that the drugs are not taken every time. The administration of Wegovy is done once a week by subcutaneous injection method. Subcutaneous injections are injections that require the use of very small needles to inject medications just under the surface of your skin.
Another great benefit of Wegovy is that the medication is specially designed for long-term use. So, it can work for long-term weight management. In one of the clinical trials for Wegovy, the effectiveness of Wegovy (semaglutide) was compared with placebos, liraglutide, and sitagliptin.
From the trials, it was reported that Wegovy (semaglutide) was superior to the others for weight loss and management and for reducing blood sugar. Another unique benefit of Wegovy is that aside from a subcutaneous injection, it also comes as an oral medication that is still taken once a week.
The oral form of Wegovy medication has the same effect as the subcutaneous injection. It also helps to reduce the level of blood glucose and to induce weight loss.
What Can Wegovy Do in Chronic Weight Management?
After the FDA's approval of this weight loss drug, the management of obesity has been changed. The use of Wegovy opens up a new avenue for improved techniques and strategies in dealing with obesity and other overweight conditions.
Even though the mechanism of action of Wegovy significantly impacts the weight reduction of the users, dealing with obesity is just like treating any other medical condition. Instead of looking at Wegovy as something that will solely solve the problem, weight management does not happen magically.
We must keep in mind that obesity also involves other physiologic and metabolic considerations. A single medication cannot combat overweight conditions and obesity. Wegovy is not enough to address this problem.
However, when this weight loss medicine is combined with strict discipline, this may be one of the keys to combat obesity. With a thoroughly formulated meal plan and properly planned physical activities, Wegovy can positively impact chronic weight management.
Who Can Take Wegovy?
Although Wegovy is great for blood sugar balance and weight loss, only some are allowed to use this medication. Suppose you are prescribed Wegovy by your doctor or healthcare provider. In that case, the medication will be delivered to you once a week.
The dosage of Wegovy you will be allowed at the initial time will be low. Consequently, the dosage will be increased slowly following your tolerance level over a couple of weeks.
It is important to know that although the United States Food and Drug Administration believes Wegovy to be effective for weight loss and reduction of blood sugar levels, not everyone should use Wegovy!
Wegovy is best used for adults with a BMI of 30 or more. That is adults who are obese. It is also for adults whose BMI is greater than or equal to 27, are considered overweight, and have some medical conditions linked to weight. Conditions like high blood pressure, diabetes, etc. These sets of adults can also use Wegovy.
If you are curious if Wegovy is for you and don't know your BMI, many online resources can help with that. You can use the online BMI calculator of the National Heart, Lung, and Blood Institute. It is easy to use. If your BMI is lower than 27, then Wegovy is not for you. Also, you can't take Wegovy if you suffer from Multiple Endocrine Neoplasia Type 2.
Multiple Endocrine Neoplasia Type 2
This is also known as MEN2. It is a very rare genetic disorder that gives rise to tumors in some important glands in the human body, including:
- Thyroid gland
- Parathyroid gland
- Adrenal gland
MEN2 can lead to other health conditions like kidney stones and high blood pressure.
How Much Does Wegovy Cost?
Since Wegovy is one of the newest and sought-after weight-loss medicine on the market today, it may be a little pricey, just like other FDA-approved weight-loss drugs.
However, our company offers Wegovy an affordable $25 a month. There is no distinct cost for Wegovy, as with most medications. The cost varies, and the price might depend on a lot of factors, including:
- Location
- Pharmacy Choice
- Insurance plan, etc.
It will help to know that there is a possibility of getting up to a 90-day Wegovy supply. If your insurance coverage eventually approves this, a 90-day supply of Wegovy can help you avoid going to the hospital or pharmacy frequently for your Wegovy.
It will help you lower the cost of Transportation. If this option makes sense and seems what you would like, then speak with your healthcare provider or doctor. You can also speak with your insurance company about it.
How Much Weight Loss Will I Get When I Use Wegovy?
Wegovy has proven its potential as a weight-loss medicine in four different clinical trials conducted. Throughout those clinical trials, more than 2,600 participants were administered Wegovy. On the other hand, approximately 1,500 participants were given placebo drugs.
These trials lasted for a total of 68 weeks. One trial involving adults without diabetes had a weight loss result of an average of 12.4% compared to the group who were given the placebo drug. The results produced by Wegovy have indeed exceeded the expectations of researchers and scientists.
It even surpassed the performance of other medications already available in the market. Despite the positive results Wegovy is giving, one user differs from the other. There are always factors that physicians must consider, and users should not see Wegovy as a magic potion that would slim them immediately. Certain factors such as lifestyle, diet, exercise, metabolism, and other physiologic functions should be considered.
Users must not expect that there will always be a 12.4% weight loss when taking Wegovy. Some patients may lose up to 20% of their body weight, while others may lose only 5%. Not all bodies respond to a medication similarly. Remember, what works for you may not necessarily work for me.
Does Insurance Cover Wegovy?
As of July 2021 manufacturer covers most of the cost; however, insurance coverage remains poor. Following the laws mandated, most companies that offer health insurance exclude the expenses for weight loss medications. Until today, major health insurance companies still see treatments for weight loss management as unimportant compared to other conditions.
These companies view these weight loss drugs as lifestyle medications, resulting in their reluctance to pay for these treatments. Since health insurance companies do not consider obesity a real-life threat and disease, most do not cover treatments for this specific condition.
Furthermore, one study aimed to examine insurance coverage according to the Medicaid and Affordable Care Act regarding drugs addressing obesity. The study revealed that only seven state Medicaid programs include weight loss drugs. In addition, out of 136 plans available, only 11% cover these products.
Cost of Wegovy with Insurance
The cost of Wegovy with insurance differs from what you would have paid without insurance. Still, the cost will vary depending on the factors we mentioned earlier and some other factors, including the number of Wegovy injection pens. If you want to know what it would cost you to get Wegovy with insurance, then speak with your doctor, a pharmacist, or your insurance company.
Is Wegovy FDA-Approved?
Wegovy was approved by the Food and Drug Administration (FDA) during the second quarter of 2021. This weight loss drug is the first medication for weight management approved since 2014. The company that manufactures this weight loss drug has been approved for use among individuals with obesity.
Since this medication is indicated for obese individuals, a particular body mass index (BMI) should be followed. An obese person has a body mass index (BMI) of 30 kg/m2. Wegovy may also be given to overweight individuals with weight-related conditions such as hypertension. If a person has a BMI of more than 27 kg/m2 but less than 30 kg/m2, they are under the overweight category.
FDA has approved Wegovy, but it also has side effects that may vary from user to user. Some common side effects of this weight loss medicine include nausea, vomiting, gastrointestinal upset, belching, bloating, and flatulence. Some users may also experience other side effects, such as diarrhea, constipation, and heartburn.
What is Included in The FDA Label of Wegovy?
The FDA label of Wegovy includes a broad range of information regarding Wegovy.
Indications and Usage
Wegovy is an adjunct therapy for chronic weight management, proper diet, and programmed physical activity in people with a BMI of 30 kg/m2. It is also indicated for overweight people with a BMI of more than 27 kg/m2 and with a weight-related condition, such as diabetes, hypertension, or hyperlipidemia.
Limitations of Use
The active ingredient of Wegovy is semaglutide, which should not be administered with other medications or products containing semaglutide and other agonists of the GLP-1 receptor. The use of Wegovy in combination with other products or medications, such as herbal products and over-the-counter medications, has not yet been proven effective and safe. It is best to use Wegovy based on your physician's advice and avoid self-medicating with other drugs.
Important Administration Instructions
Before initiating the treatment using Wegovy, the patients must first be taught the proper administration technique. Before every injection, users must inspect the medication physically. A thorough inspection of the drug before the injection is also included in the instructions and recommended dosage, schedule of dose escalation, and dosage form and strengths.
Contraindications
The FDA label of this weight loss medicine included severe hypersensitivity reactions such as angioedema and anaphylaxis towards any product containing semaglutide as a contraindication for Wegovy. Family history or personal history of thyroid malignancies is also included in the contraindications, along with patients diagnosed with Multiple Endocrine Neoplasia Type 2.
Warnings and Precautions
The FDA label of Wegovy also considered the warnings and precautions in taking the weight loss medicine. This includes patients with hypoglycemia and hypersensitivity reactions.
Clinical Trials Experience
Aside from the common facts about this weight loss drug, the results of the clinical trials involving Wegovy have also been included.
Drug Interactions
The FDA label stated that drug interactions might occur when Wegovy is used with another insulin secretagogue or other oral hypoglycemic medications. Wegovy may also lead to delayed emptying of gastric contents and affect the absorption of other oral drugs.
Use in Specific Groups
The use of Wegovy among special populations such as lactating mothers, pregnant women, pediatric and geriatric age groups, and patients with hepatic and renal impairment was also placed on the FDA label.
Overdose
There have been reports of Wegovy overdose combined with other GLP-1 receptor agonist medications. Patients may experience side effects, including nausea, vomiting, and hypoglycemia. Physicians must immediately give appropriate treatment for the symptoms being experienced.
Clinical Pharmacology
The FDA label also included thorough details about the mechanism of action of Wegovy, along with its pharmacodynamics and pharmacokinetics.
Others
Other information, such as the weight loss medicine's nonclinical toxicology, is included. Clinical studies, supply, storage and handling, patient counseling information, and a medication guide that contain basic and easy-to-understand information are also included.
How Do I Take My Wegovy?
According to the FDA label of Wegovy, this weight loss drug must be administered once every week on the same day. It may be administered at any time of the day with no needed dose adjustments. Users may also take Wegovy with or without meals.
This medication is administered using an injection via the subcutaneous route. Sites where you may inject Wegovy, include the upper arm, abdomen, or thigh. If the user misses a dose and the next scheduled administration of the medication is more than 48 hours away, they may take Wegovy as soon as they remember it.
On the other hand, if they miss a dose and the next scheduled dose is less than 48 hours, do not take the missed dose anymore. Continue the dosing on the scheduled day. If the user misses two straight doses for two weeks, continue the dosing as scheduled, or they may consult their physician to start the weight loss medicine following the escalation schedule. This may help lessen the side effects experienced in the gastrointestinal system when Wegovy is reinitiated again.
As per the recommended dosage of Wegovy, it can be initiated with a dosage of 0.25 mg injected via a subcutaneous route once every week. Users should follow the dose-escalation schedule to avoid gastrointestinal side effects.
Wegovy Side Effects
All drugs and medications have their unique benefits and their side effects as well. The same applies to Wegovy, which has side effects similar to all GLP-1 hormone agonists. These side effects are gastrointestinal and improve or get better as your body adjusts and adapts to the medication.
However, you must know that while taking Wegovy, you should avoid other medications containing Semaglutide or any other agonist of GLP-1. Agonists of the GLP-1 hormone are known for reducing the sugar level in your blood. Too much can reduce the blood sugar level to dangerous lows and increase the intensity of the side effects you might experience.
This can also occur when you take more than your doctor or healthcare provider prescribed. Side effects of Wegovy vary in different categories. There are serious side effects, mild side effects, and common side effects.
Common Side Effects
The common side effects of Wegovy (Semaglutide) include:
- Nausea and Vomiting
- Diarrhea
- Stomach aches and pain
- Constipation
- Extreme tiredness or fatigue
- Headache
- Mild to moderate heartburn
Mild Side Effects
The mild side effects of Wegovy (Semaglutide) include:
- Acid reflux
- Stomach bloating
- Irritation of the stomach
- Dizziness
- Burping
- Flatulence
- Gas
Serious Side Effects and Adverse Reactions Some Wegovy side effects are serious and should be reported to a doctor or a healthcare provider immediately after they are noticed. They include:
- Adverse reactions and allergies, including hives or itching, rashes on the skin, swelling of the lips, tongue, and face
- Change in vision
- Rapid heartbeat
- Gastroenteritis
- Hair loss
- Viral gastroenteritis
- Issues with the gallbladder
- Low blood sugar in Type 2 Diabetic Patients
- Inflammation of the pancreas
- Lumps or swelling in the neck
- Difficulty swallowing
- Difficulty breathing
- Lump or swelling on the neck
- Depression
- Suicidal thoughts
Contraindications of Wegovy
As we mentioned earlier, although Wegovy is great for weight loss and reduction of blood sugar levels, it should not be used by everyone. This set of people includes:
- People who have a family or personal history of medullary thyroid cancer
- People with Multiple Endocrine Neoplasia Syndrome Type 2
- People are known to be hypersensitive to Wegovy (semaglutide) or any components of the medication.
Some Health Problems Caused by Wegovy
Some health problems are caused by Wegovy, especially in people who have a very high risk of developing these conditions in the first place. If you are at risk of any of the following health conditions, avoid Wegovy (semaglutide) to avoid these problems.
Thyroid C-cell Tumors
Studies have shown that when the dosage of Wegovy is increased or used for a longer time, it can increase the risk of thyroid cancer. This was observed while studying rats; it is unknown if it also occurs in humans. So, make sure to inform your doctor if you notice any changes, including:
- Lumpiness and hoarseness in the neck
- Shortness of breath
- Difficulty breathing and swallowing
Hypoglycemia
When blood sugar is too low, it can lead to confusion, extreme tiredness, irritation, headache, unconsciousness, sweating, etc.
Pancreatitis
Call your health provider or doctor if you develop stomach pains that accompany nausea, vomiting, and fever and radiate to your back. It is also best to discontinue Wegovy at this point.
Diseases of the Gallbladder or Gallstones
This can lead to jaundice (yellowing of the eyes or skin) and pain in the upper abdomen.
Diabetic Retinopathy in Type 2 Diabetes
Taking Wegovy when living with type 2 diabetes can lead to changes in your vision. If you notice any, inform your doctor or healthcare provider.
Acute Kidney Injury
You might develop acute kidney Injury and experience symptoms or signs like changes in the color and quantity of your urine.
If you are pregnant or hoping to get pregnant in the next two months, do not take Wegovy (semaglutide).
Wegovy vs. Other Weight Loss Drugs and Therapies
The FDA has also approved several weight-loss medicines that may be used as an alternative to Wegovy. Last 2017, Ozempic, a GLP-1 receptor agonist, was approved for the adjunct management of diabetes. Aside from diabetes, it is also used as an adjunct for chronic weight management.
Just like Wegovy, it is also given once weekly but in a lower dosage. Saxenda is another weight loss drug that is used for weight management. This drug helps users lose an average of 5% of their body weight. As mentioned before, Wegovy helps users lose up to 12% of their average weight.
Rybelsus and ozempic are semaglutide molecules and cause significant weight loss; however, they are designated weight-loss products. The primary goal is to help control diabetes while also helping with weight loss.
Should I tell my Doctor Before Using Wegovy?
Clinical trials and studies have shown that Wegovy is safe and effective for long-term weight loss. Still, like most medications, it has its side effects and should not be used by everyone. Contact your doctor or healthcare provider, or reach out to us to know if Wegovy is the right choice for weight management.
Conclusion
Wegovy is here to change the game and start a new avenue in chronic weight management. With its safety and efficacy, it can indeed be an addition to the strategy to combat the public health issue of obesity.
About The Author
Ahmet Ergin, MD, FACE, CDCES, ECNU Endocrinology 2260 Palm Beach Lakes Blvd. Ste 212 Unit #7 West Palm Beach, Florida
Who is Dr. Ergin?
Dr. Ahmet Ergin is an endocrinologist interested in and passionate about diabetes care. Dr. Ergin earned his medical degree with honors at Marmara University School of Medicine in Istanbul, Turkey, and completed his internal medicine residency and endocrinology fellowship at Cleveland Clinic in Cleveland, Ohio.
He is a board-certified Internal Medicine, Endocrinology, Diabetes, and Metabolism Physician; a certified diabetes education specialist, the author of The Ultimate Diabetes Book; and the Founder of the SugarMD youtube channel.
He practices in Port Saint Lucie, FL as an endocrinologist physician. Disclaimer: Any information on diseases and treatments on this website is for general guidance only and must never be a substitute for the advice your doctor or other qualified healthcare professional provides. Always seek the advice of your physician, health provider, or other qualified healthcare professional's advice with questions regarding your health.
REFERENCES:
Christou, G. A., Katsiki, N., Blundell, J., Fruhbeck, G., & Kiortsis, D. N. (2019). Semaglutide as a promising anti-obesity drug. Obesity reviews: an official journal of the International Association for the Study of Obesity, 20(6), 805–815. https://doi.org/10.1111/obr.12839 Scheen A. J. (2019). Le médicament du mois. Le sémaglutide, agoniste des récepteurs du GLP-1 en injection sous-cutanée hebdomadaire (Ozempic®) [Semaglutide, once weekly GLP-1 receptor agonist (Ozempic®)]. Revue medicale de Liege, 74(9), 488–494. Wilding, J., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., Kushner, R. F., & STEP 1 Study Group (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. The New England journal of medicine, 384(11), 989. https://doi.org/10.1056/NEJMoa2032183
Written By Dr. Ahmet Ergin
457 total articles
Meet Dr. Ahmet Ergin, a highly skilled and dedicated endocrinologist with a passion for diabetes care. Dr. Ergin earned his medical degree with honors from Marmara University in Istanbul. He completed internal medicine residency and endocrinology fellowship at Cleveland Clinic. Dr. Ergin is board-certified in Internal Medicine, Endocrinology, Diabetes, and Metabolism due to his vast medical expertise. He's a certified diabetes educator, author of “The Ultimate Diabetes Book,” and founder of “the SugarMD YouTube channel.” Dr. Ergin offers exceptional diabetes care to his patients in Port Saint Lucie, FL, helping them manage effectively. For a closer look into his insights and experiences, connect with Dr. Ahmet Ergin on LinkedIn, Instagram, and YouTube.”
Disclaimer: These statements have not been evaluated by the Food and Drug Administration. Information on this website isn't intended to treat, cure or prevent any disease. Discuss with your doctor and do not self-treat.
Products